Magnesium Hydroxide
Magnesium hydroxide (Mg(OH)2) is a chemical treatment for wastewater, used for hydrogen sulfide (H2S) control in wastewater collection systems and at treatment plant headworks processes. Magnesium hydroxide is an alkali source that works by increasing the pH of the treated wastewater. The chemical is typically added to the wastewater as a slurry at a collection system pump station or at an injection point immediately upstream from a pump station.
Magnesium Hydroxide Addition Systems
H2S dissolves in water and disassociates in accordance with the following reversible reaction: H2S « HS– + H+
At a pH of 7, approximately 50% of sulfides are dissolved H2S gas, while the other 50% are hydrosulfide (HS–) ions, as shown in the following graph. As pH rises, the concentration of hydrosulfide ions rises. The hydrosulfide ions cannot escape from solution into the atmosphere. Raising the wastewater pH also affects microbial activity in the pipe slime layer. Sulfate reducing bacteria (SRB) are most active at a pH of 6.5 to 7.5. Increasing the pH beyond this range can reduce the SRB’s sulfide reducing capabilities. At pH 9, approximately 98% of dissolved sulfides can not be released.
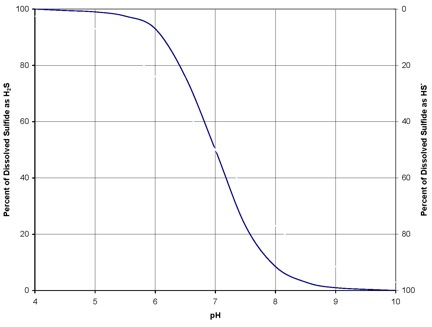
Since the relative portions of H2S and HS– are dependent on pH, the objective of feeding magnesium hydroxide is to increase the pH of the wastewater to 8.0 – 8.5. At this pH, nearly all of the sulfides will be in the HS– form and cannot be released as H2S.
Magnesium hydroxide is completely safe to handle and is not classified as a hazardous chemical. The slurry is stored in chemical storage tanks and needs to be continuously mixed to prevent coagulation and settling in the tank. Secondary containment is not required; however, the slurry will freeze at approximately 32oF so precautions to prevent freezing in the tank, piping and chemical pump(s) must be taken.
Magnesium hydroxide can build up in wetwells if the wetwell is not thoroughly mixed.
Applicable Treatment Processes
Force mains, gravity sewers. WEA has found that it is particularly effective on forcemains with long detention times and low flows.

